Science Seen Physicist and Time One author Colin Gillespie helps you understand your world.
A Better Battery
Here’s some good news: A better battery may soon be on its way to a device near you. (There’s bad news too but it will have to wait.) Building better batteries is all about smaller, lighter, cheaper, faster charging, longer lasting . . . and most of all getting batteries to hold more charge. This key attribute is measured as the energy-to-weight ratio or energy density, in watt-hours per kilogram.
Better batteries are based on chemistry. Lithium is the leading chemical of interest. It is the lightest metal. A lithium cell can give a high electrical potential (more than 3 volts) and, in theory, the highest energy density. So for thirty years inventors have been trying different to build better rechargeable lithium batteries. It’s mostly about finding better chemistry with different lithium compounds. This is where the latest innovations lead.
From the day Alessandro Volta invented them in 1800, batteries have always had three parts. Electrical current flows from a positive electrode (anode) to your device and back to a negative electrode (cathode) where it completes the circuit through an (often liquid) electrolyte. Today’s lithium-ion batteries use a wide range of lithium compounds in both the anode and the electrolyte. Charging the battery drives positive lithium ions onto the cathode. The chemistry of the cell is such that—left to go their own way—these ions would rather go to the anode. This creates the cell’s voltage, and it drives the current when you use the battery. All parts of a typical lithium-ion battery are sealed inside and under pressure. The best lithium-ion cells hold about 200 watt-hours of energy per kilogram.
By contrast, the cathode of a lithium-air cell is exposed to the air and the electrolyte is usually solid. During discharge the cathode takes up oxygen from the air to turn lithium ions into lithium oxide. Charging gives the oxygen back. In theory a lithium-air cell’s energy density can be high but in practice its performance faces many challenges.
Enter a British team of chemists. They give the lithium-air cell some serious tweaks. Their electrolyte includes some water. 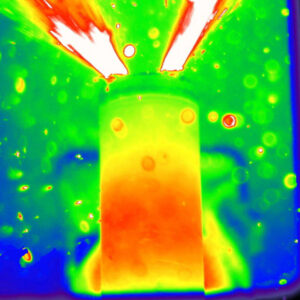 During discharge their cathode takes up oxygen from the air and water from the electrolyte to turn lithium ions into lithium hydroxide. Their design packs tiny lithium hydroxide crystals closely. The big advantage of their cell comes from adding a low level of lithium iodide to the electrolyte. It’s the iodide that does the crucial work of tightly packing lithium hydroxide crystals without clogging up the pores and ‘suffocating’ the cell with other reaction products. The result is a very high energy density—over 5,000 watt-hours per kilogram—and a cell that seems to solve most of the lithium-air system’s problems with, they say, ‘impressive rechargeability’. You could even hope for a commercial version to be tucked inside your next smartphone.
During discharge their cathode takes up oxygen from the air and water from the electrolyte to turn lithium ions into lithium hydroxide. Their design packs tiny lithium hydroxide crystals closely. The big advantage of their cell comes from adding a low level of lithium iodide to the electrolyte. It’s the iodide that does the crucial work of tightly packing lithium hydroxide crystals without clogging up the pores and ‘suffocating’ the cell with other reaction products. The result is a very high energy density—over 5,000 watt-hours per kilogram—and a cell that seems to solve most of the lithium-air system’s problems with, they say, ‘impressive rechargeability’. You could even hope for a commercial version to be tucked inside your next smartphone.
So what’s not to like? Well, the bad news is the flip side of the good news, that same high energy-density that drives your high-performance device and lasts all week. Lithium-ion batteries sometimes suffer spectacular thermal runaway. Physical damage or overheating leads to sudden release of the concentrated energy as heat. Recently another British team used high-speed x-ray photography to capture an image of the resulting explosion. And there’s the rub, as Shakespeare says. The new lithium-air cell will be much better at exploding too. In fact the energy density in your new battery may exceed that of the explosives standard, TNT.
Sources:
Tao Liu et al. (2015), “Cycling Li-O2 batteries via LiOH formation and decomposition”, Science, Washington: AAAS, vol. 350, p. 530; http://www.sciencemag.org/content/350/6260/530.full
Other Materials:
Donal P. Finegan et al. (2015), “In-operando high-speed tomography of lithium-ion batteries during thermal runaway”, Nat. Commun., p. 6.
Image credit:
Donal Finegan, University College London; https://www.ucl.ac.uk/electrochemical-innovation-lab/people/phd-students/donal_finegan

No comments yet.